Abstract
Background: The heterogeneity of MDS and its outcomes has led to the evolution of treatment modalities that are guided by patients’ Revised International Prognostic Scoring System (IPSS-R) risk. Supportive care is frequently given to patients with lower risk MDS, while hypomethylating agents (HMAs) are used in higher risk patients. Despite the use of HMAs and other therapies, outcomes remain poor and there is a need for novel treatments in higher risk patients. Additionally, real-world evidence is limited in the community practice setting. The aim of this study was to understand patient characteristics, treatment patterns, and OS of patients with higher-risk MDS (intermediate/high/very high risk [IPSS-R score >3]).
Methods: This is a retrospective analysis of adult patients with treatment-naïve, newly diagnosed MDS in The US Oncology Network with an IPSS-R risk > 1.5 between 1/1/2011 and 1/1/2021. Study data were sourced from structured electronic healthcare records for patients under community-based outpatient care through 7/1/2021. Patient characteristics and treatment patterns were assessed descriptively, with the Kaplan-Meier method used to assess OS.
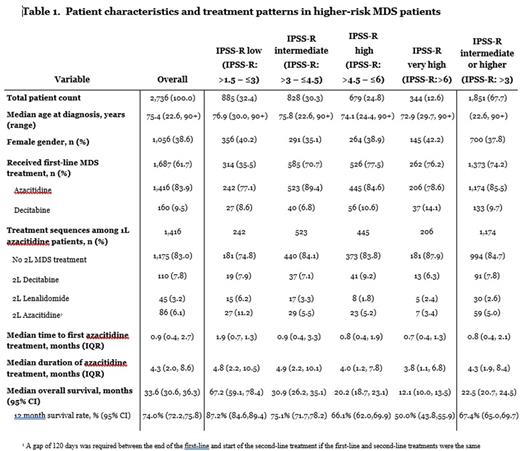
Results: Overall, 2,736 patients met eligibility criteria (median age 75.4 y, 38.6% female). Distribution of IPSS-R for low (>1.5 - ≤ 3), intermediate (>3 - < 4.5), high (>4.5 - < 6) and very high (>6) risk was 32.4% (n=885), 30.3% (n=828), 24.8% (n=679), and 12.6% (n=344) of patients, respectively (Table). In total, 61.7% (n=1,687) of all patients received 1st-line HMA treatment, compared to 35.5% (n= 314) of lower-risk (<3) and 74.2% (n-1,373) of higher-risk (>3) IPSS-R patients during the study period. Among higher-risk pts, azacitidine (AZA) was the most common 1st-line therapy [85.5% (n=1,174)] followed by decitabine [9.7% (n=133)]. 84.7% of pts treated with 1st-line AZA did not advance to 2nd-line MDS treatment. Median (IQR) time to first AZA treatment (months) was twice as long among lower-risk patients compared to higher-risk patients (1.9 [0.7, 10.3] vs. 0.8 [0.4, 2.1], respectively). Median (IQR) AZA treatment duration (months) was 4.3 (1.9, 8.4) in higher-risk patients, with 4.9 (2.2, 10.1), 4.0 (1.2, 7.8), and 3.8 (1.1, 6.8) for pts with intermediate-, high- and very-high risk MDS. Median (95% CI) OS (months) in higher-risk patients was 22.5 (20.7 - 24.5), and ranged from 30.9 (26.2-35.1), 20.2 (18.7-23.1) and 12.1 (10.0-13.5) among intermediate-, high- and very-high risk IPSS-R scored patients with MDS.
Conclusions: To our knowledge this is the largest analysis to date examining real-world treatments patterns and outcomes in higher-risk MDS patients. The results suggest that patients with higher-risk MDS continue to experience short treatment duration with HMAs and suboptimal outcomes. Future studies can investigate the outcomes of novel treatment options for higher-risk MDS in the community oncology setting.
Disclosures
Lyons:Incyte Corporation: Consultancy, Other: Research Consulting Services; Texas Oncology/The US Oncology Network: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company, Other: Leadership; Celgene: Honoraria. Cheng:AbbVie: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Moore-Schiltz:Inovalon: Current Employment; Ontada: Current equity holder in private company, Ended employment in the past 24 months. Shi:Ontada: Current Employment. Svensson:AbbVie: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Bui:AbbVie: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Hutti:AbbVie: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. McNeill:AbbVie: Current Employment, Current equity holder in private company. Mearns:Genentech: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company; Roche: Current equity holder in publicly-traded company. Yellow-Duke:Genentech: Current Employment, Current equity holder in private company, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Zackon:Incyte: Consultancy, Other: Research Consulting Services to Incyte; Ontada: Current Employment; Epizyme: Consultancy; Morphosys: Consultancy.
OffLabel Disclosure:
Venetoclax is a BCL-2 inhibitor that is FDA approved for multiple indications. Venetoclax is not currently approved for the treatment of myelodysplastic syndrome. Azacitidine is FDA approved for continued treatment of adult patients with acute myeloid leukemia who achieved first complete remission or complete remission with incomplete blood count recovery following intensive induction chemotherapy and are not able to complete intensive curative therapy.
Author notes
Asterisk with author names denotes non-ASH members.